Precious
Metals Refining By Solvent Extraction
General
If
a compound (= solute) is dissolved in a liquid (water) and the
solution is brought into contact with a second immiscible liquid (=
solvent, maybe a hydrocarbon etc.), then a part of the solute is
transferred to the second liquid phase by a force called the chemical
potential. The physical-chemical process of transferring the solute
between the bulk of the two immiscible liquid phases is called
solvent extraction.
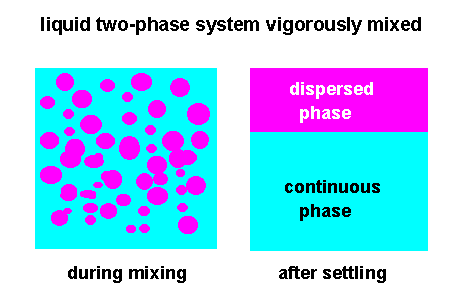
During intensive
mixing of both liquids in the course of time the mass transfer of the
solute between the two liquid phases deminishes and finally at very
long contact times vanishes; an equilibrium
is encountered. The time necessary to reach 90% of the
equilibrium is characteristic for a given solute/solvent system and
is in the range of seconds up to hours for technically relevant
systems. This time is a function of the product kt
a ; (mass transfer coefficient) x (interface area/liquid
volume).
In technical equipment equilibrium is never reached to a
hundred percent; 90 to 99% is considered to be sufficient.
Commonly
for practical process layout the equilibrium is characterized by a
distribution coefficient which is defined as the ratio D =
(all species of solute in organic phase)/(all species of solute in
aqueous phase). D is dependent on the initial concentration of the
solute and the concentration of other reaction components in
question. The distribution coefficient is independent of phase
flow conditions and somewhat characteristic for a given
solute/solvent/aqueous system. It is one of the key parameters in the
design of a solvent extraction process.
|
|
After the
calculated mixing time in a batch process or residence time in a
continuous mixing process has elapsed, mixing of the two liquid
phases is stopped and both liquid phases are allowed to coalesce
and settle by gravity or centrifugal force.
The rate of
coalescence is highly depending on the viscosity, density and
interfacial tension of the liquids and drop size of the dispersed
phase.
|
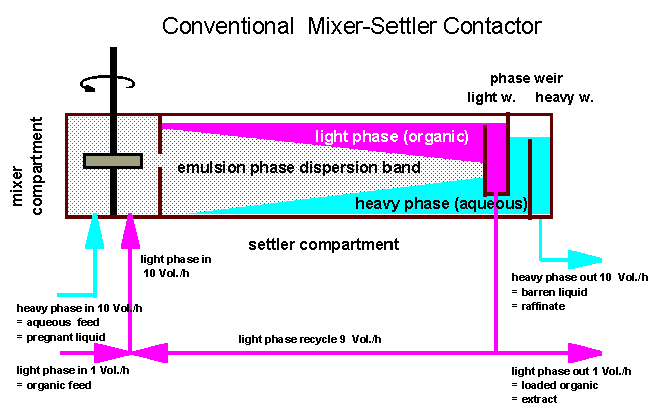
The basic equipment
to perform a continuous mixing and coalescence process on a technical
scale is called a mixer-settler.
It is often used in
laboratories as an ideal tool for basic system design of continuous
solvent extraction processes because it offers reproducable phase
flow and contact times. The device comprises a continuously fed and
stirred mixing compartment (see sketch below) and a gravity settler
compartment where the liquids are allowed to separate. At the end of
the settler two individual weirs care for good separation of the
liquid bulk phases. The flow capacity of a mixer-settler is reached
when the emulsion phase dispersion band overflows the light phase
weir or underflows the heavy phase weir.
In technical
mixer-settler devices the volume of the settler compartment is often
10-fold the size of the mixer compartment to provide sufficient
settling time even for systems with low coalescence rates (in case of
high viscosity and/or low density and/or low interfacial tension).
For a good
performance of the settler the liquid phase ratio organic/aqueous
in a mixer-settler should be kept close to one.
To adjust a
definite feed ratio of organic/aqueous- independent of optimum
internal phase ratio - an external or internal phase recycle can be
installed. In the sketch of a conventional mixer-settler below a feed
ratio of O/A = 1/10 is shown.
Hence such a process would
concentrate a solute in the loaded organic by a factor of 10 if the
distribution coefficient is high enough.
In
nearly all practical cases it is necessary to have more than one
mixer-settler unit. Repeated mixing and settling can provide a high
concentration and purity of the solute especially if the
distributuion coefficient is low or even close to one. In multistage
solvent extraction mixer-settler batteries often are built as
contiguous boxes which can save a lot of space.
In
applications where a very high number of stages is required
theoretically and in large scale industrial application of more than
100 m3/h solvent extraction is preferably run in continuous
countercurrent column equipment (reciprocating plate, rotating
disc, stirred column).
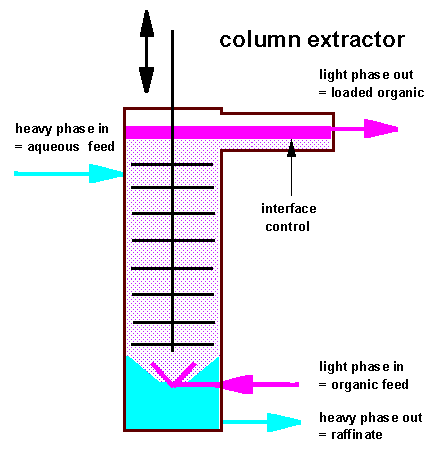
In
column extractors there are no longer discret compartments where
mixing and settling of the liquids occurs but new formation of
droplet with "fresh interface" and coalescence of droplets
prevails allmost in each section of the column in the same extent.
|
On the right the
principle scheme of a perforated plate reciprocating column is
shown. Organic feed enters the bottom part of the column through a
spray nozzle or ring distributor system and is allowed to coalesce
at the top in a separate horizontally mounted settling device to
increase the interfacial surface.
|
|
The control of the
hydraulic stability of solvent extraction columns is much more
difficult to maintain than in mixer-settlers.
In some cases if
solvent extraction is governed by an irreversible chemical reaction
of the solute with other components in the solvent the process can be
performed in a stirred tank reactor as a batch
mixer-settler process.
Please note that the
feed for a solvent extraction process containing the solute in
question is not necessarily always aqueous. There are lots of
examples in industrial organic synthesis where crude organic product
solutions have to be treated with aqueous solutions or water for
purifying. Moreover the solute is not always the valuable target
component in a solution, it might just be an impurity which has to be
removed.
Reactive
Extraction
In cases if solvent extraction is enabled by a
chemical reaction (at the interphase of aqueous/organic or in one or
both of the bulk phases) or if a prevailing chemical reaction is
accelerating the basic physical mass tranfer, this process is called
reactive extraction. The item was first used and introduced
into literature in 1981 by Werner Halwachs in his publication
"Reaktivextraktion" (Habilitationsschrift, Universität
Hannover, Fachbereich Chemie, 1981).
Metal Extraction
As far as solvent extraction of metals or precious metals is
concerned an additional chemical, a so-called extractant, is
necessary to form a chemical compound (= complex) with the solute and
thus make it soluble in the organic phase.
Furthermore additional
chemicals, so-called modifiers, are used to enhance
coalescence of the emulsion and increase the solubility of metal
complexes formed in the solvent to suppress formation of solids which
may lead to a total collapse of the process by clogging..
Solvent extraction
of metals or precious metals is used in refineries for
the
removal of individual precious metals or group of metals from a
pregnant liquor comprising other pgm, base metals and /or salts (=
separation).
the
removal/reduction of impurities from crude solutions of individual
precious metals or a group of metals (= refining)
In most cases a
complete industrial solvent extraction cycle for metal extraction
comprises four consecutive solvent extraction steps in order to win
concentrated or purified solute and recover solvent and
extractant - otherwise the process would be uneconomic and
environmentally hazardous; those subprocesses are:
extraction;
transfer of the metal into the organic phase by chemical reaction
with the extractant
scrubbing;
removal of coextracted material/metals or excess acid etc.
(optional)
stripping;
transfer of the metal back into a second pure aqueous phase for
winning or further processing
solvent
make-up; treatment of the organic by a third aqueous phase for
purification of solvent or extractant; removal of crud or
degradation products; topping with fresh organic (optional)
In
large scale industrial solvent extraction process where raffinates or
barren liquids are drained to effluent treatment plants, the
solubilities of solvents and extractants in the aqueous liquids
are of superior importance; during solvent extraction system
design in the lab phase extractant/solvent combinations must be
selected comprising as low aqueous solubility as possible; otherwise
those chemicals might be harmfull to the environment.
|
Below some examples of industrial importance concerning
precious metal solvent extractions
|
|
|
|
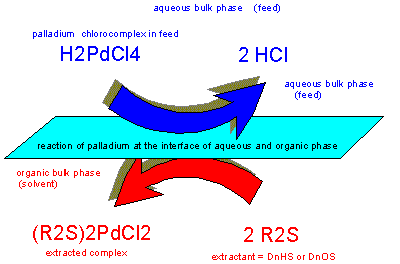
Alkyle
sulfides are selective for gold, silver and palladium. In pgm
refinery circuits they can help to separate palladium from
platinum, iridium and rhodium if no gold or silver is present in
solution or separated before.
Ruthenium
and osmium as well as strong oxidants should not be present either
to prevent degradation of the valuable extractant.
|
|
|
|
|
|
|
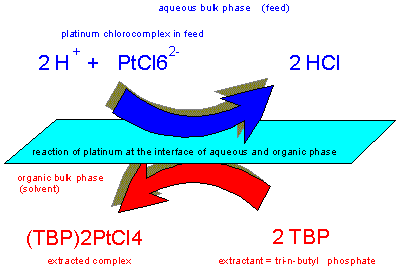
Tri-n-butyl
phosphate is a versatile low-cost extractant highly resistant
against oxidants and reducing agents for the separation of
platinum and iridium from complex base metal solutions and
rhodium.
|
|
|
|
|
|
|
|
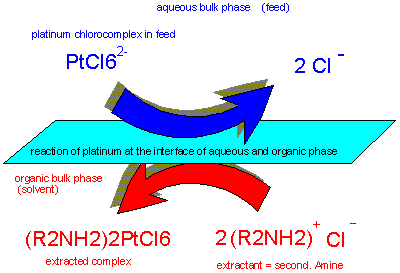
Secondary
amines can provide good separation of platinum from cathionic base
metals in dilute aqueous solution in order to produce CPA etc.
|
|
|
|
|
|
|
Would you like to
head deeper into the theory of reactive extraction ?





